Introduction
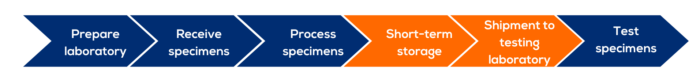
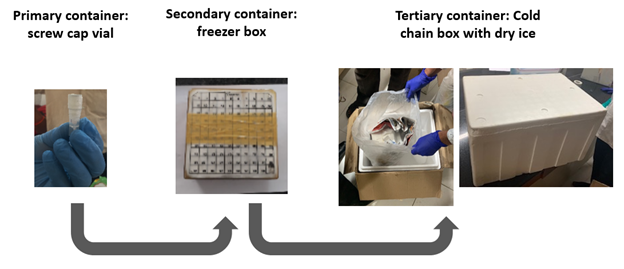
This module describes activities occurring from the time the specimens reach the laboratory following collection to the point of testing, including specimen receipt, processing, storage, and shipment.
Learning Objectives
To receive, process, store, and transport specimens collected as part of a serosurvey
Topics Covered in this module
Technical and logistical demands of processing, storage, and shipment of specimens
Note: Refer to below modules for related information