Introduction
Laboratory testing for biomarkers requires standardized techniques for specimen collection and processing, high quality testing assays, and meticulous procedures for data management and analysis. Specimens can be venous blood, capillary blood obtained through a finger or heel prick, and oral fluid or saliva.
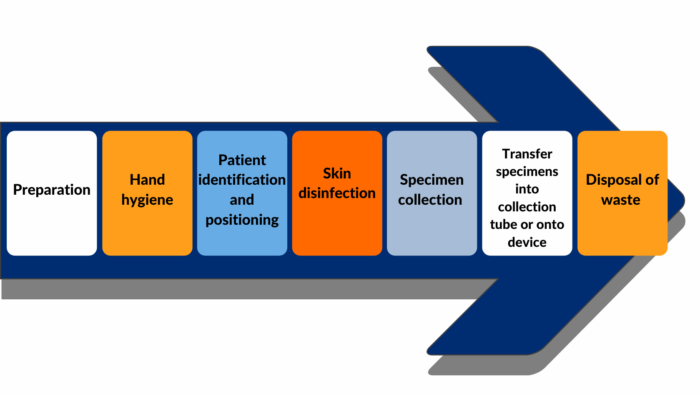
This module describes the procedures for collecting high quality blood specimens in the community and transporting specimens to the laboratory. This includes considerations for all the steps of blood collection, as depicted in Figure 1.
Learning Objectives
To collect high quality blood specimens in the community and transport to the laboratory
Topics covered in this module
Technical and logistical demands of collecting, processing, and storing a blood specimen in the community and transporting the specimen to the laboratory.
Note: Refer to the below modules for related information