Introduction
This module describes the process of testing specimens in the laboratory and key considerations for seroprevalence studies.
Learning Objectives
- Provide an overview of laboratory assays and specimen management in the laboratory
- Understand key aspects required in a laboratory testing protocol
Topics covered in this module
- Operational considerations in handling and managing specimens
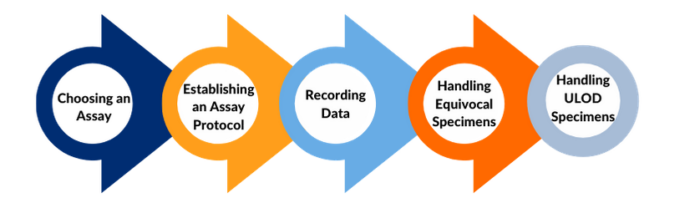
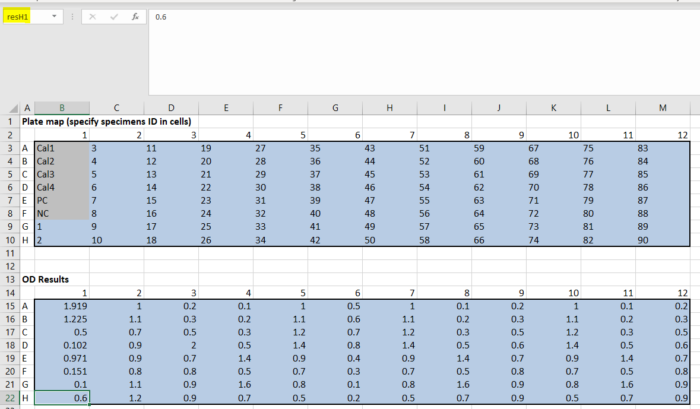
- Considerations for what to include in laboratory testing protocols
Note: Refer to below modules for related information
- Specimen Collection
- [MODULE – Specimen processing, storage, and shipment]
- Quality assurance for assays and interpretation of results