Introduction
This module describes sampling residual blood specimens from biorepositories or health facilities to be used for serosurveys.
Learning Objectives:
- Define residual blood specimens and identify potential benefits of this design for serological surveillance
- Describe the ethical, data, and operational considerations prior to accessing a biorepository or health facility specimens
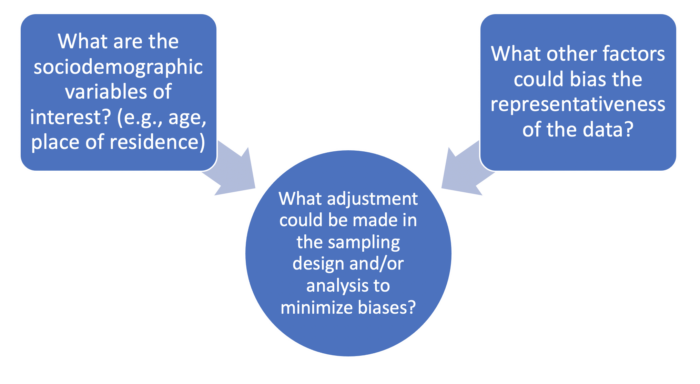
- Describe considerations for data analysis when using residual blood specimens, particularly their representativeness of the target population
Topics covered in this module
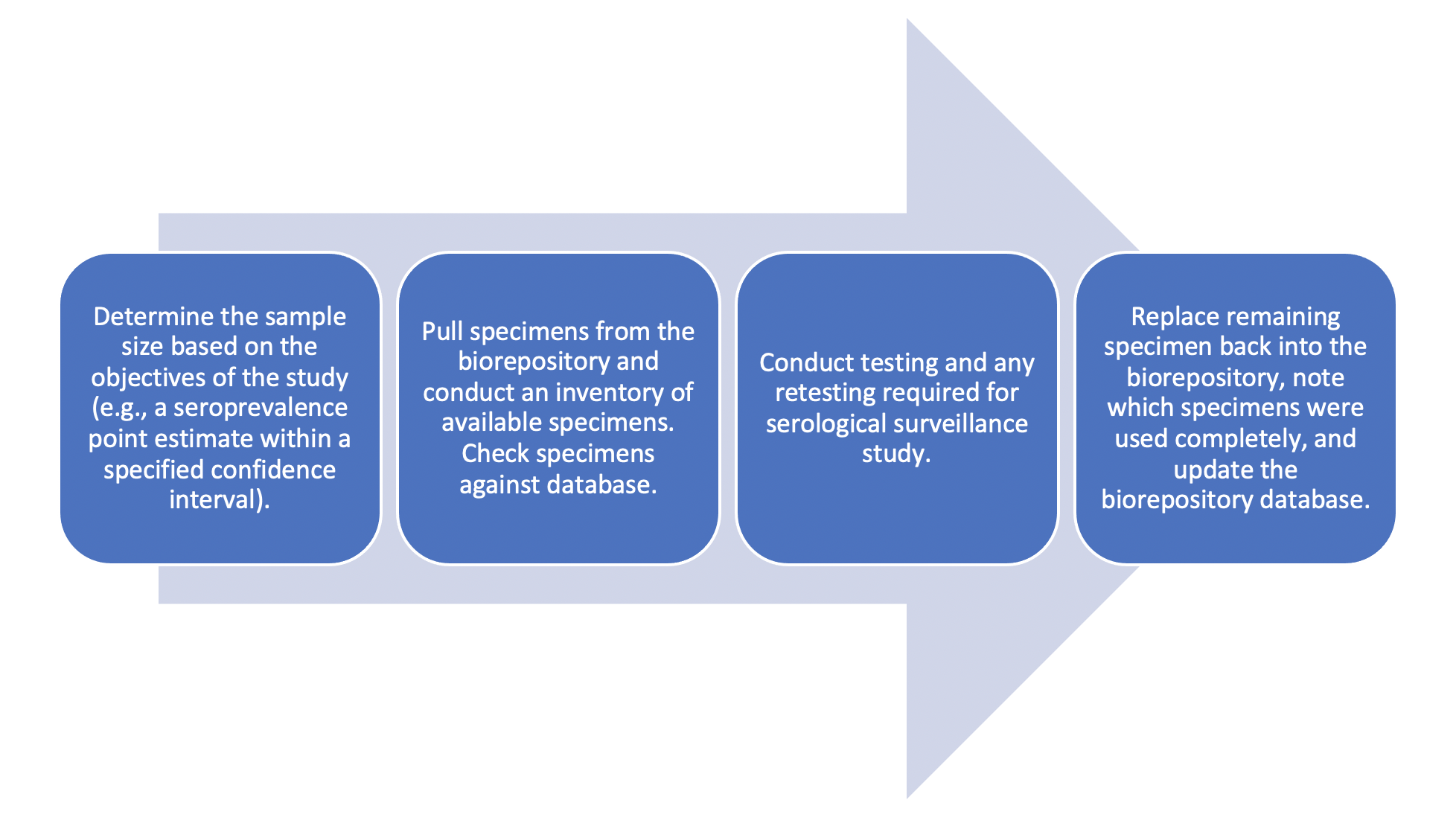
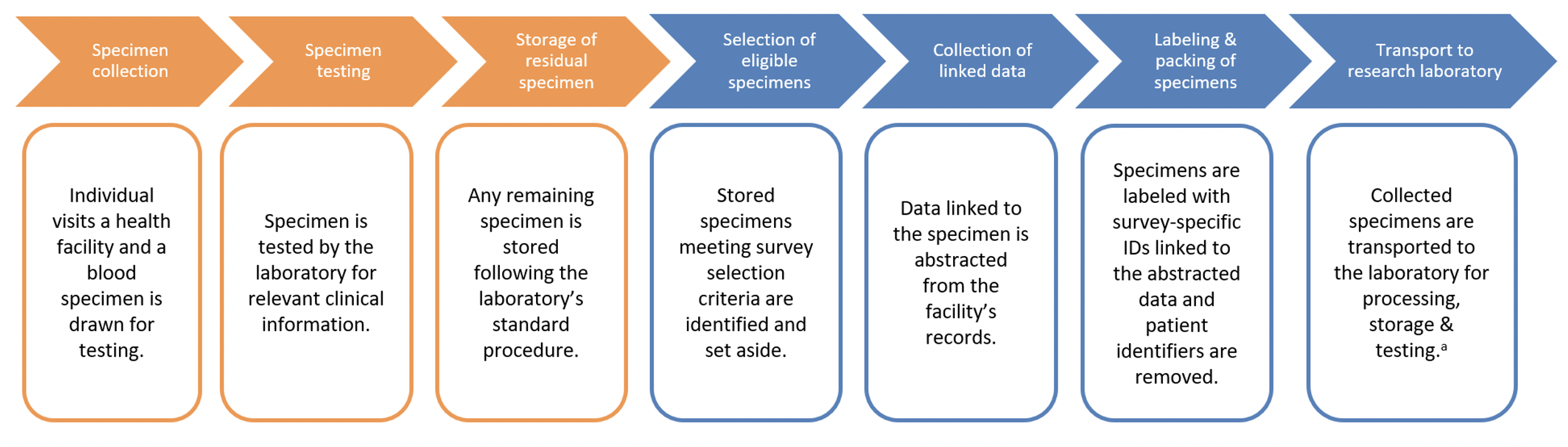
Technical and analytic considerations for accessing and using blood specimens from a biorepository for serosurveys.
Note: Refer to these modules for related information
- Assay protocols and laboratory data management
- Using existing clinical specimens from facility or laboratory